After the appearance of potential ALD-like symptoms (ataxia, spasticity, deafness, visual deficits, behavioral disturbances), patients are encouraged to undergo diagnostic testing. Neuroimaging techniques such as Magnetic Resonance Imaging (MRI), Computed Tomographic (CT) scans, and Positron Emission Tomography (PET) scans are valid techniques for examining cerebral demyelination in patients with X-linked ALD and usually provide the first lead to diagnosis in relation to symptoms.
The following images are from a 3 year case report of a 24-year old male diagnosed with ALD with a 10 year history (Volkow et al. 1987). Imaging was first conducted in 1984 where the patient had progressive loss of visual acuity. Ten months later (1985), the patient returned and neurological examination revealed progression of visual defect and decreased proprioception. 8 months later, the patient experienced acute psychosis, delusions, almost complete visual loss, hallucinations, ataxia, and progressive loss of memory and attention span.
This first image depicts a CT scan of the patient with decreased density in white matter (shown by decreased contrast) over the three year case study.
These images show an MRI scan of the patient in 1985, and then in 1986. There is further edema (excess swelling) into white matter tracts (right image) and demyelination.
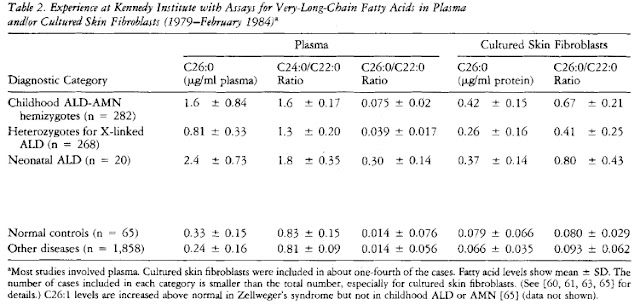
After clear observation of brain damage and demyelination, a plasma, fibroblast, or red blood cell assay is the most widely used and convenient step for a definitive diagnosis. Measurements of very long chain fatty acids levels (VLCFA), mainly the fatty acid hexacosanoate (C26:0), in red blood cell phospholipids or total plasma lipids are taken. Elevated C26:0 levels, as wells as elevated C24:0/C22:0 and C26:0/C22:0 ratios indicate a peroxisomal disorder in which these fatty acids are unable to undergo oxidation and may indicate ALD. A fibroblast assay may also take place to detect elevated VLCFA levels. The table below shows increased C26:0 levels and ratios in Childhood Adrenoleukodystropy-Adrenomyeloneuropathy (ALD-AMN), Heterozygotes for X-linked ALD (mothers - carriers), and neonatal ALD (Moser et al. 1984):
Additionally, a family history of the disease is suggested. It is recommended that mothers (the carriers) also have their plasma and fibroblast levels of hexacosonoate examined for a more definitive diagnosis of X-ALD in their male offspring. It has been observed that female heterozygotes also exhibit elevated C26:0 levels and ratios and may potentially exhibit some symptoms of ALD. Furthermore, due to increased chance of false negatives in plasma and fibroblast measurements, a mutational analysis is recommended for all potential carriers. The table below shows elevated C26:0 levels in diagnosed heterozygotes for X-linked ALD (Moser et al. 1984):
Flowchart of recommended actions of diagnosis (Shimozawa 2011):
Nevena V.
References
Moser HW, Mahmood A, Raymond GV (2007) X-linked adrenoleukodystrophy. Nature:Clinical Practice. 3(3):140-151.
Moser HW, Moser AE, Singh I, O'Neill BP (1984) Adrenoleukodystrophy: Survey of 303 Cases: Biochemistry, Diagnosis, and Therapy. Ann Neurol. 16: 628-641.
Shimozawa N (2011) Molecular and clinical findings and diagnostic flowchart of peroxisomal diseases. Brain and Development. 33: 770-776.
van Geel Bjorn, Assies J, Wanders RJA, Barth PG (1997) X linked adrenoleukodystrophy: clinical presentation, diagnosis, and therapy. Journal of Neurology, Neurosurgery, and Psychiatry. 63: 4-14.
Volkow ND, Patchell L, Kulkarni MV, Reed K, Simmons M (1987) Adrenoleukodystrophy: Imaging with CT, MRI, PET. The Journal of Nuclear Medicine. 28(4): 524-527.




No comments:
Post a Comment